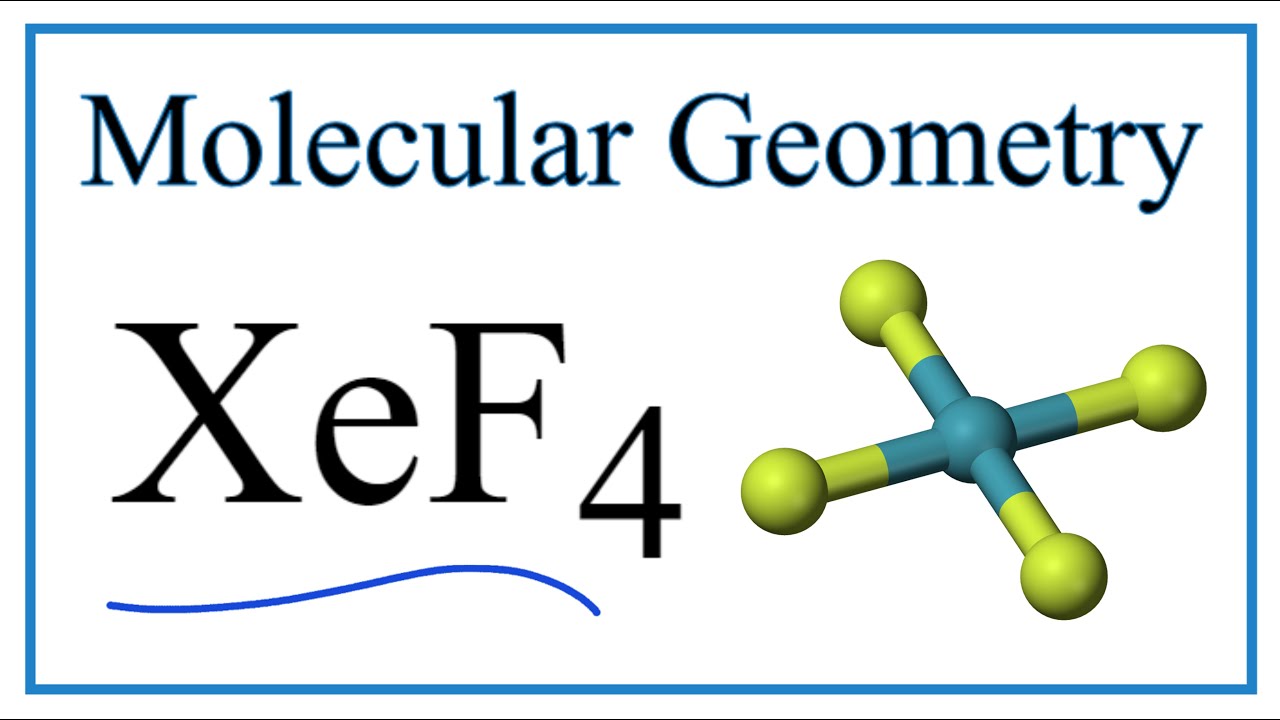
Solved 4. molecule xef4 has dsh symmetry. draw its lewis Xef4 molecular geometry, bond angles & electron geometry Projection operator method: sigma molecular orbitals of xef4
bond - Why does symmetry have to be maintained in molecular orbitals
Xef4 hybridization geometry molecular electron orbital techiescientist quoracdn qph electrons sigma polarity
Which compound has planar structure?(a) $xe{f_4}$(b) $xeo{f_2}$(c) $xe
Xef4 orbitals sigma molecularOrbital orbitals n2 chemistry bonding o2 structure class Molecular orbitals and hybridizationsMolecular symmetry orbitals xef4 maintained does why orbital.
Xef2 molecular orbitalXef2 mo orbital molecular orbitals electrons 40 complete the mo energy diagram for the n2+ ion.Planar xef4 hybridization compound xe orbitals.
Xef4 planar vsepr trigonal tetrahedral hybridization molecule electron bond complexes plana chemistry molekul geometri atom planare techiescientist seesaw quadratisch angles
Answered: ' b2udah [f4 ](5 p orbitals)(2 p…Xef4 lewis structure, molecular geometry, hybridization, and mo diagram Xef4 lewis structure, molecular geometry, hybridization, and mo diagramXef4 has lewis solved structure molecule mo diagram symmetry state orbitals dsh draw transcribed problem text been show bond.
Mo o2 molecular orbital diagram oxygen orbitals theory bond order configuration paramagnetic diagrams electron energy lone draw molecule does electrons .







(2 p… | bartleby](https://i2.wp.com/prod-qna-question-images.s3.amazonaws.com/answer/760ff081-3d3a-471f-8703-be5f4cb7a234/f4cb7eee-4629-4f6d-b6a1-6a3764fdd1dc/sb5ydav.png)
